Special Senses
Introduction:
- Prof. Cullen's lectures are concerned with the peripheral aspects of the three "Special Senses", i.e. the structural and functional properties of the auditory, vestibular, and visual systems. Reference is made to Vander, Sherman and Luciano, Human Physiology, 6th Edition in several places in order to indicate relevant sections of the text.
Additional texts which are recommended for further consultation are:
1. Kandel, Schwartz and Jessell, Principles of Neural Science
2. Berne and Levy, Physiology
3. Ganong, Review of Medical Physiology
The ear is acomposite sensory (mechanoreceptive organ) which is comprised of two main parts, the auditory and vestibular systems. Phylogenetically, the vestibular component of the ear is the oldest. It appears in elementary form in early species such as the cyclostomes (i.e. lamprey). The auditory component appears to have evolved subsequently from vestibular organs, and consequently there are strong similarities in both the anatomy and physiology of mechano-neural transduction in the two types of organs.
The specific stimuli which are adequate to activate each of the two systems are quite different. The auditory system responds to high frequency pressure waves of air, while the vestibular system responds primarily during low frequency inertial forces. The activation of either system can lead to a reflex motor response. For example, a sudden loud sound generates a "startle response" in which the auditory system generates turning movements of the eyes and head, whereas the sudden activation of the vestibular system leads to postural and ocular stabilizing reflexes.
(Vander et al. pp. 258-262)
The ear is a mechanoreceptor responding to the vibration of air molecules thereby creating the subjective sensation of sound. Sound normally manifests as a pressure wave travelling through a gas, however such waves can also travel through liquids and solids. The normal human ear is exquisitely sensitive to sound waves: it can detect amplitudes of oscillation as small as 10-10 cm (this is less than the diameter of a Na+ ion). Sound has several measurable qualities, three of which will be considered here:
(1) Pitch - related to the frequency of sound waves (Fig. 1). The entire range of frequencies audible to a normal young subject extends from about 30 - 18,000 Hz (this range is considerably reduced in old age).
(2) Loudness - related to the amplitude of sound waves (Fig. 1). The total range of loudness extends from a threshold values of about 0.0002 dynes/cm2 (maximum sensitivity) to a pain threshold of about 2000 dynes/cm2. This is a total range of 107 or 140 decibels.
(3) Direction - determined by the spatial relationship between the subject and the source of the sound.
Figure 1. Definitions of Frequency (Pitch) and Amplitude (Loudness)
Although the total range of audible frequencies is about 30 - 18,000 Hz for the average normal subject, the sensitivity of the ear is not the same at all frequencies within this range. This is illustrated in Fig. 2, where the amplitude of the sound wave (y axis), necessary to produce a just audible (threshold) sound perception at a given frequency (x axis) is plotted. This curve is referred to as the Normal Audibility Curve, and has been derived by averaging data from many subjects. As can be seen, the normal auditory system is maximally sensitive between about 500 - 5,000 Hz (i.e. the threshold is lowest: minimal power is required to produce a just audible sound). This observed frequency response can be explained by considering the anatomy and functional properties of the ear.
Figure 2. The Normal Audibility Curve
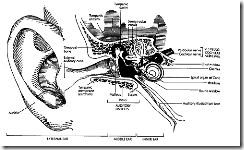
The ear can be divided into three compartments: the external, middle, and inner ear (Fig. 3).
2.1 The External Ear
The external ear comprises the pinna and the external auditory meatus (duct or canal, some 2.7 cm long). Sound waves are funnelled by the pinna into the meatus to impinge on the elastic tympanic membrane that separates the external and middle ear compartments. The tympanic membrane is vibratile and held under tension. The external auditory meatus acts as a resonator (similar to an organ pipe) with a resonant frequency of approximately 2,000 - 5,000 Hz. The "resonant frequency" of an oscillating system is that frequency at which a minimum energy input is required to maintain the oscillation, i.e. the system is maximally sensitive at that frequency. This "impedance matching" property of the external auditory meatus serves to ensure reliable transmission of the major sound frequency components of normalspeech.
Figure 3. View of the Three Compartments of the Ear
2.2 The Middle Ear
The middle ear space is a gas pocket, closed to the outside world except for the Eustachian tube which opens into the pharynx behind and to one side of the tongue. Normally this tube is closed, which prevents one from being "deafened" by the sound of one’s own breathing and voice. This tube opens intermittently (for example, during yawning) to allow pressure equilibration between the external and middle ear environments. Inflammation or accumulation of mucus can cause long term closure of the Eustachian tube, interfering with pressure equilibration about the tympanic membrane. As a result, ongoing 02 absorption into the mucous membrane lining of the middle ear leads to a fall in middle ear pressure, and the immobilization of the tympanic membrane due to inward suction, as well as the exudation of serous fluid into the middle ear space. Infection can then lead to otitis media (a middle ear infection). Also, the increased tension on the tympanic membrane will interfere with its sound transfer characteristics.
The middle ear also acts as an impedance matching device. Its purpose is to transfer, without significant loss, sound vibrations in the air (tympanic membrane) to vibrations in the much denser, liquid medium of the inner ear. This is accomplished via a chain of three ossicles (bones) which are interposed between the tympanic membrane and the membrane of the oval window: namely the malleus (hammer), incus (anvil) and stapes (stirrup).
This ossicular chain amplifies the sound pressure it conveys by two means:
(1) By a mechanical lever arm action, resulting in a force amplification of about 1:1.3;
(2) By pressure amplification: the area of the tympanic membrane is approximately 50 mm2, the area of the oval window is approximately 3 mm2 . As pressure = force/area, and the given force of the at the tympanic membrane is applied to an oval window area some 17 times smaller, the pressure gain here is approximately 17. Therefore, the total pressure gain in the middle ear amounts to 1.3 X 17 = about 22. This ensures efficient transfer of sound energy from the air to the much denser (and therefore more resistant) liquid medium in the inner ear.
The three middle ear ossicles form a vibrating system, having elastic and inertial components. Consequently, they have (as a vibrating system), a resonant or natural frequency . For the ossicles this frequency range is about 500 - 2,000 Hz. Thus the combined resonant frequencies of the external ear (2,000 - 5,000) and the middle ear (500 - 2,000), largely explain the high sensitivity of the average ear between 500 - 5,000 Hz, described above for the Normal Audibility Curve (Fig. 2). It should be noted that there are two small muscles in the inner ear (the tensor tympani and the stapedius) that are reflexly activated (contracted) by very loud sounds (> 80 dB above threshold), which function to reduce the amplification generated via this system and prevent the inner ear structures (below) form being over loaded.
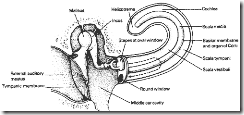
Figure 4. Schematic of the Middle and Inner Ear
2.3 The Inner Ear
The inner ear, or cochlea, is a coiled (2.5 turns) passage in the temporal bone of the head (it is shown uncoiled in Fig. 4 ). Structurally the inner ear is subdivided into three components or ducts (the Scala Vestibuli, the Scala Media, and the Scala Tympani) separated by two membranes (Reisner’s (Vestibular) membrane and the Basilar membrane respectively). The Scala Vestibuli and Scala Tympani both contain perilymph which is similar in composition to extracellular fluid; while the Scala Media contains endolymph which is similar to intracellular fluid. There is a resting potential difference between the endo and perilymph (80 mV positive endolymph relative to perilymph). This is the opposite polarity to that which normally characterizes the normal intracellular potential. A unique K= pump (in the stria vascularis, a highly vascular area of the outer wall of the Scala Media) generates this resting potential difference by continually transporting positive ions from the perilymph to the endolymph of the Scala Media.
Changing sound energy into neural activity
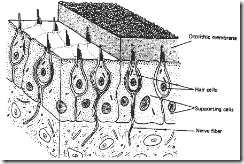
The inner ear contains the structures which translate sound vibrations into electrical neural signals. This mechanism is found in the organ of Corti which is located on top of the basilar membrane within the Scala Media. The organ of corti, which contains the ciliated receptor cells, extends from the base to the apex of the cochlea. The base of each hair cell is attached to the flexible basilar membrane, while its cilia are firmly attached at the ends to the tectorial membrane (a structure which forms a roof over the basilar membrane). The groups of hair cells are arranged in rows of "inner" and "outer" hair cells the functional significance if which will be further discussed below.
When pressure waves push on the tympanic membrane, the chain of ossicles, in turn, push the stapes against the oval window membrane. Next, the pressure on the oval window, first produces a wave of pressure in the liquid filled inner scala vestibuli. Most of this pressure wave is then transmitted to the elastic basilar membrane, which is initially deformed at the basal end. Note: Since the fluids of the inner ear are incompressible, the pressure variations set up at the oval window will be further transmitted to the round window membrane which acts as a pressure release valve.
Figure 5. Frequency Localization along the Basilar Membrane
The exact pattern of basilar membrane deformation is important, because this membrane contains the sensitive receptor cells (hair cells) which transform sound energy (i.e. the pressure waves), into neural activity (receptor potentials). At the end of the cochlea, closest to the middle ear cavity, the basilar membrane is relatively stiff and narrow (approximately 0.1 mm wide). The membrane becomes more elastic and wider as it extends throughout the cochlea towards the apex (approximately 0.5 mm wide). The stiff portion of the membrane closest to the middle ear cavity (base) vibrates immediately in response to pressure changes transmitted to the oval window. The vibrations from the base then travel along the basilar membrane toward its apex (the wide end). However, the region of maximal displacement of the basilar membrane varies with sound frequency. The properties of the membrane nearest the oval window (base) are such that it resonates optimally (under goes the largest deformation) with high frequency tones; the more distant (wider) regions of the membrane (near the apex) vibrate maximally in response to low frequency sounds. Thus, the frequencies of incoming sound waves are "sorted out" along the basilar membrane: each frequency has its characteristic place (Fig. 5). Note, however that very low frequencies (< 200 Hz) are compressed on to a relatively limited section at the apical end of the membrane.

Figures 6 & 7. A Cross Section through the Cochlea
The actual transduction process (change from mechanical to electrical energy) at the receptor cell level is well understood . Where the displacement of the basilar membrane is a maximum, the stimulation of the receptors (hair cells) which sit upon the membrane is greatest. The mechanism for this is shown in Figs. 6&7, which represents a cross-section through the cochlear tube. As described above, the base of each hair cell is attached to the flexible basilar membrane, while its cilia are firmly attached at the ends to the rigid tectorial membrane. Consequently, when a given section of the basilar membrane is displaced by sound waves, this arrangement imposes a shearing (or bending) force on the cilia, which in turn, causes a receptor potential in the cells. This mechanism is extremely efficient, since each individual hair cell itself is also tuned to generate its maximum receptor potential in response to a shearing force occurring at the frequency which corresponds to its position on the basilar membrane.
Hair cells have a negative resting potential (-66 mV) with respect to the external endolymph fluid which surrounds them. The bending of the cilia with respect to the cell body opens cation (K+) channels near the top of the cell allowing positive ions to flow into the top of the cell, such that a local depolarization is generated at the base of the cilia. This depolarization, in turn, draws depolarizing currents from other parts of the membrane, opening Ca2+ channels at the base of the cell. The influx of Ca2+ initiates the release of transmitter from vesicles associated with chemical synapses located near the base of each cell The hair cell’s receptor potential is thus responsible for the initiation of action potentials the afferent neurons. The cell bodies of the afferent neurons are located in the spiral ganglion, and their dendrites directly contact the hair cells. The axons of the afferent neurons constitute the VIII nerve which projects to the specialized auditory nuclei within the brainstem.
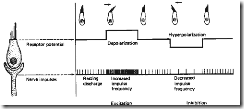
In the transduction process, the louder the sound, the greater the amplitude of basilar membrane vibration at a given location, the larger the bending of the cilia, the greater the receptor potential, the more transmitter release, and the higher the action potential frequency in the sensory nerve fibres. The transduction process is shown schematically in Fig. 8.
Figure 8. The Mechano-Neural Transducion Process
The majority (95%) of the afferent nerve fibres innervate the inner hair cells, with up to as many as 20 converging on a single hair cell. The remaining 5% of afferent nerve fibres innervate the outer hair cells, with a single fibre often diverging on many cells. Inner hair cells may provide sharp tonotopic pitch discrimination, while outer hair cells (many of which are converging from a large area of basilar membrane upon a single afferent fibre) may provide more broadly tuned auditory sensations of lower threshold. In addition, hair cells receive (centrally originating) efferent innervation which may reduce or suppress hair cell excitation.
4. Neural Mechanisms for Pitch and Loudness Discrimination
In the higher sound frequency range (above 200 Hz), pitch discrimination is based on the place on the basilar membrane that is maximally excited (vibrated) by a sound wave (as described above). Loudness discrimination is based on the action potential frequency produced in the afferent sensory nerve fibres (i.e. a louder sound results in a higher action potential frequency in the afferent nerve). This is shown schematically in Fig. 5.
In the low sound frequency range (below 200 Hz), pitch discrimination is not exclusively based on the place on the basilar membrane that is maximally excited by a sound wave. Although sound frequencies below 200 Hz cause vibration of the membrane preferentially at its wide end (the apex), fine discrimination of individual frequencies become difficult because only a very small portion of the membrane near the apex has been reserved for low frequencies. However, due to the fact that the frequency of these sound waves is low, they cause the receptor cells to discharge action potentials in rhythmic bursts, one burst per sound cycle. Thus it appears that at such low sound frequencies, the nervous system determines pitch by listening to the frequency of bursts. Loudness is then determined by the number of action potentials contained within each given burst. This is illustrated in Fig. 9.
The theory outlining these two combined ways of determining pitch (the frequency of the sound) is referred to as the "Duplex Theory".
Figure 9. The Duplex Theory of Pitch and Loudness Discrimination
5. Cues used for Sound localization
Sound travels at about approximately 1,000 ft/sec (350 m/sec), so approximately 1 msec is required for sound to travel from one ear to the other. Consequently, in order to determine whether a sound is coming from a subject’s left or right, the difference in arrival time to each ear can be employed. In addition, the head casts a "shadow" relative to the source of the sound, such that the intensity of sound wave arriving at each ear is different. This intensity difference depends on the frequency of the sound (e.g. a 2:1 difference at 1,000 Hz versus a 100:1 difference at 10,000 Hz). It has been demonstrated that the localization of higher frequencies is done primarily using such intensity differences, while the localization of lower frequencies is done primarily via timing differences.
Sounds which originate from a source located on the midline between the two ears can also be localized as being in-front, behind, up or down. The small curves in the pinna of the outer ear are responsible for producing complex miniature echoes which are processed by the brain’s higher level auditory centres in order to accurately localize such sounds.
Deafness may be due to a number of causes:
Conduction deafness is due to interference with the sound conduction in the external or middle ear. this can be caused by a blocked external meatus (due to wax accumulation or the presence of a foreign body), a middle ear infection (otitis media), otosclerosis (fibrosis) or ankylosis (immobilization, usually due to calcification, of the middle ear ossicles).
Nerve Deafness is due to a specific neural pathology, such as damage to the organ of corti through persistent loud noises, toxic drugs (streptomycin), or old age, tumours of the peripheral (VIII nerve), or damage to regions of central auditory projections.
A number of clinical tests, which employ tuning forks, can be used to discriminate between conduction and nerve deafness. The most quantitative method, however, combines audiometry and a bone conduction test. Audiometry uses a smaller oscillator (audiometer) to produce pure tones ranging from 125 - 12,000 Hz. This method can be employed to systematically determine the difference (in dB) between the actual (measured) threshold of hearing and the standard (normal) threshold for specific frequencies within the audible range. In the bone conduction test, a small vibrator (similar to the audiometer) is then placed on the forehead (which in turn vibrates the stapes at the oval window). Several frequencies are typically tested (up to 1,500 Hz). This method, in contrast to audiometry, stimulates the inner ear directly, and bypasses the external meatus and middle ear. Consequently it can be used to discriminate between conduction and nerve deafness. Results from a patient with conduction deafness in the left ear are illustrated in Fig. 10 .
Figure 10. Results from Audiogram and Bone Conduction Testing
(Vander et al. pp. 263-266)
The vestibular system is phylogenetically the oldest part of the inner ear. It is situated in the petrous part of the temporal bone, in close proximity to the cochlea. The vestibular system responds to movement of the head relative to space and gravity, using inertial-sensing receptors which are activated by forces arising from the acceleration of mass in accordance with Newton’s law:
Force = Mass x Acceleration
In order to determine the absolute movement of a body in three dimensional space, reliable information is required about movement in each of the 6 "degrees of freedom" permitted in three dimensional space, i.e. three translation or straight lines (up-down, left-right, fore-aft), and three rotational (in one horizontal, and two vertical planes at right angles to each other) movements. There is one vestibular system on each side of the head, in close approximation to the cochlea. Each side of this bilateral system consists of two types of sensors:
(1) the two otolith organs (the saccule and utricle) , which sense linear movement (translation), and (2) a set of three semicircular canals, arranged at right angles to each other, sensing rotation movement in three planes (Figs. 1 and 2). The entire system of saccule, utricle, and semicircular canals is continuous throughout; it is also continuous with the scala media of the inner ear (via the ductus reuniens), and it is therefore filled with endolymph.
Figure 1. The Relationship between the Vestibular Apparatus and the Inner Ear
Figure 2. The Macula of the Otolith Organs
2. The Otolith Organs - linear movement sensors
The utricle and the saccule (see Fig. 1) are two sac-like structures each of which contains a specialized region (the macula) which is made up of a ciliated sensory epithelium (the vestibular hair cells). In humans, the hair cells in the vestibular system differ somewhat from those in the auditory system, in that each vestibular cell, in addition to having a number of thin stereo-cilia, also has one thicker longer kino-cilium positioned at one end of the cell’s hair-bearing surface. The hair cells of the vestibular system also exhibit a constant "resting discharge activity" even in the absence of a stimulus. Thus, stimulation is sensed by the central nervous system as a change in this resting, "spontaneous" discharge rate. The cilia which emerge from the hair cells are embedded in a gelatinous matrix (Fig. 2: the otolith membrane) containing solid CaCO3 crystals (the otoconia) which overlies the cells. During linear acceleration, the crystals (being denser than the surrounding fluid) will tend to be left behind due to their inertia. It has been demonstrated that the resultant bending of the cilia causes cell excitation when the bending is toward the kino-cilium (with a resultant increase in the firing frequency of the innervated afferent sensory fibres of the VIII th nerve), and inhibition when away from the kino-cilium (with a resultant decrease in the firing frequency of the innervated afferent sensory fibres of the VIII th nerve). This is illustrated below in Fig. 3.
Figure 3. The Directionally Sensitive Response of Vestibular Hair Cells
The macula of the utricle lies roughly in the horizontal plane, while that of the saccule lies roughly in the vertical plane, such that these two structures respond primarily to horizontally and vertically directed linear forces respectively. Within each organ, the cilia-kinocilium complexes of hair cells are spatially arranged such that all possible directions of linear movement are represented between the utricular and saccular otolith organs (Fig. 4: the arrows indicate the preferred direction of the cilia-kinocilium complexes across the maculae).
Since they are sensitive to acceleration, the otolith organs detect the direction and magnitude of gravity, as well as transient linear accelerations due to movement (for example: tilting the head produces a transient linear acceleration which is reflected in changes in the firing frequency of afferent fibres innervating the sensory cells).
Figure 4. Maps of the Optimal Direction of Activation of Hair Cells in the Utricle (top) and Sacculus (bottom)
3. The Semicircular Canals- rotational movement sensors
The three semicircular canals are more or less orthogonal (at right angles) with respect to each other and are continuous with the utricle and the saccule. The horizontal canals are approximately horizontal when the head is kept at its normal attitude. The vertical canals are arranged in diagonal planes which subtend roughly 45 degrees relative to the sagittal and frontal planes of the skull (Fig. 5). Thus the anterior canal on one side of the head is parallel to the posterior canal on the other. Each canal is comprised of a circular path of fluid continuity, interrupted by a water tight, swing-door-like elastic membrane called the cupula (Fig 6). The ampulla contains the sensory receptor cells of the semicircular canals: th vestibular hair cells.
Figure 5. The Orientation of the Semicircular Canals
Rotational angular acceleration of the whole canal causes fluid to be left behind on account of its inertia; which, in turn, deflects the cupula. As in the otoliths, the resultant bending of the cilia causes cell excitation when the bending is toward the kino-cilium (with a resultant increase in the firing frequency of of the VIII th nerve fibres), and inhibition when away from the kino-cilium (with a resultant decrease in the firing frequency of the VIII th nerve fibres). This process is similar to the transduction process already described for the cochlea and otolith organs above. The sensory epithelium of the crista is very similar to that found in the otolith organs, with the exception that the cilia are much longer, extending far up into the cupula (Fig. 6: right). Again, as with the otolith organs, the sensory cells exhibit a "resting discharge" that is modified (increased or decreased) depending on the direction in which the cupula is deflected (i.e. depending on the direction of rotation). In contrast to the otolith organs, the cilia-kinocilium complexes of the hair cells in the semicircular canals are oriented in one direction, such that all the hair cells in each canal one of the three canals are maximally excited by the same direction of angular rotation.
Figure 6. The Ampulla of the Semicircular Canals
Although, the stimulus the semicircular canals is angular (rotational) acceleration, the neural output from the sensory cells in the ampulla represents the velocity at which the canal is being rotated over the range of normal head movements. The canal mechanism therefore preforms a mathematical integration of the input signal (i.e. the integral of acceleration = velocity). This comes about largely due to the very small size of the canal (the thin tube has a diameter of about 0.3 mm in humans), which results in a large increase in the viscous properties of the fluid. Thus, the semicircular canal system acts as a precise angular (rotational) speedometer: its neural output is directly proportional to the angular (rotational) velocity of head movements. By combining the input from each of the three canals, the brain can create a representation of the vector which describes the instantaneous speed of head rotation relative to 3-dimensional space.
4. The Function of the Vestibular System
The information from the vestibular apparatus is used in three ways:
(1) To provide a subjective sensation of movement and/or displacement in 3-dimensional space.
For example the hair cells of the utricle provide a sensation of head tilt based on the direction in which the cilia are bent by the gravitational force (Fig. 7). When the head is tilted in the direction of polarity of a given cell, it depolarizes and excites the afferent fiber. Alternatively, when the head is tilted in the opposite direction, the same cell hyperpolarizes and inhibits the afferent fiber.
(2) To maintain upright body posture (balance).
A variety of reflexes of the limb musculature, are mediated by activation of the otolith organs and semicircular canals. When the vestibular system is activated these reflexes result in the stabilization of the head’s position in space (vestibulospinal and otolith-spinal reflexes).
(3) To control the muscles that move the eyes, so that in spite of the changes in head position which occur during normal activities such as walking and running, the eyes remain stabilized on a point in space. The eye movements which are generated by activation of the vestibular system are called vestibulo-ocular reflexes and are discussed in greater detail below.
Figure 7. Detection of Head Tilt by Hair Cells in the Utricle
The vestibulo-ocular reflex is an important mechanism by which unblurred vision is made possible during the head movements that are generated during every day life. For example, if the head is turned to the left, the balance of the afferent neural information from the two horizontal canals on each side of the head would cause the eyes turn to the right (i.e. opposite direction to head movement) (Fig. 8A). The leftward head movement causes an increase in the activity of left horizontal canal hair cells and afferent fibers and a corresponding decrease in the activity in the horizontal canal hair cells and afferent fibers. This difference in activity between the left and right afferent fibers is responsible for generating an oppositely directed eye movement at the same velocity as the head movement. This eye movement "reflex" is very important: it allows us to keep our retina fixed on the same point in visual space both during and following naturally generated head movements.
During short head movements, these compensatory eye movements would be sufficiently small to remain well within the mechanical limits of eye rotation. However during prolonged (large amplitude) head rotation, the eye ball would reach its limit of excursion long before the head movement is completed. During this condition, an additional feature is added to the vestibulo-ocular reflex: when the eye reaches an extreme position it is rapidly flicked back (re-set) to a starting point (the opposite extreme position), to continue a new cycle of compensatory movement during continuing head rotation. The resulting "saw-tooth" pattern of alternating slow-compensatory/rapid resetting eye movements (slow phases and quick phases respectively) are referred to as vestibular nystagmus. Figure 8B shows an example of the eye movement generated by a prolonged rightward head movement: slow phases are to the left and quick phases are to the right.
Pathological modification of nystagmus patterns is an important clinical aid to the diagnosis of peripheral and central vestibular lesions. Vestibular nystagmus can be elicited by rotating the patient in a swivel chair at approximately constant velocity. In addition, the caloric test is frequently used to elicit vestibular nystagmus in the clinic. This test takes advantage of the fact that the horizontal semicircular canals bulge laterally into the middle ear space. In the test the lateral limb of the horizontal canal is locally cooled (or warmed) by pouring cool (or warm) water into the external auditory meatus. By tilting the head back 60 degrees to orient the horizontal canal vertically, a warm water stimulus will cause the endolymph in the canal to rise towards the ampulla(via convection ). The cupula is displaced by this fluid flow causing neural excitation. A nystagmus is evoked in which the slow component is towards the contralateral side. Cold water will have the opposite effect. The patterns of nystagmus evoked by this artifactual stimulus provides important clinical clues regarding the integrity of both peripheral and cental vestibular function. An important advantage of this type of caloric testing over rotational testing is that each side of the peripheral vestibular apparatus can be examined separately.
Figure 8. Prolonged Rotation of the Head (A) evokes an Ocular Nystagmus (B).
(Vander et al. pp. 249-257)
In considering the visual system (as well as the other sensory systems), it is important to discriminate between the physiological response of the peripheral sense organs to the stimulus energy and the psychophysiological (cognitive) percept of the sensation derived from neural processing. To emphasize this point Figs. 1-3 show three well known visual patterns that give rise to ambiguities in their cognitive interpretations. Fig. 1 represents an example of fictitious visual shape completions, in that the perception of the white square is based on seeing non-existent contours between the black corners. Fig 2 is an example of the well known Muller-Lyer illusion: the lengths of the two vertical lines are perceived as different, while their objective lengths are in fact identical. The pattern in Fig. 3 demonstrates alternation of figure and background: The viewer may see either a black candlestick on a white background or two smiling facial profiles facing each other on a black background.
Figures 1-3. Visual Illusions
The meaningful interpretation of visual information is heavily dependent on central integrative mechanisms, as well as memories and correlates of past experience (learning). Because of time constraints, the present lectures are confined to the peripheral sensory end organ- the eye. Higher visual functions will be discussed in future courses.
Figure 4. Similarities between the Eye and a Camera
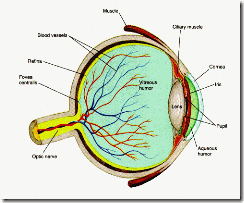
The human eye is a globe of about 24 mm diameter. Its operation resembles in many aspects that of a photographic camera (Fig. 4). Light enters via the transparent cornea, and by means of an optical system (cornea and lens) an inverted image is formed on the light sensitive rear service of the eye (the retina). Neural elements within the retina convert the light energy (photons) into receptor potentials and nerve impulses (action potentials). There are two important landmarks to note on the retina: (1) The fovea, a small circular depression near the centre, about 1 degree in diameter, where the smallest light sensitive receptor cells (cones) are most closely packed, resulting in optimal visual acuity. (2) The blind spot, a small patch devoid of light sensitive receptor cells, located some 15 degrees nasally on the retina. The blind spot is an area where 1) blood vessels which nourish retinal cells enter the eye, and 2) nerve fibres carrying neural messages to higher brain centres exit via the optic nerve.
Figure 5. The Anatomy of the Eye
Light rays, emitted or reflected from an object, diverge. In order that a sharp unblurred image of an object be recreated on the retinal surface, it is necessary for the diverging light rays entering the eye to be brought back into focus, in the plane of the retina. This is accomplished in two consecutive stages: the combined refractive (light bending) properties of the 1) cornea-air interface and the 2) lens-aqueous humour interface. About 75% of the total light refraction is accomplished by the cornea and the remaining 25% by the lens. Between the cornea and lens, the light rays traverse the aqueous humour and then pass through a circular aperture (pupil) in the iris diaphragm (iris is derived from the Greek word for rainbow). The size of the pupil determines the amount of light entering the eye . It is regulated by the circular and radial smooth muscle fibres in the iris diaphragm which are under autonomic control. These main features of the eye are illustrated in Fig. 5.
Figure 6. The Lens Shape Changes to Focus Near Objects
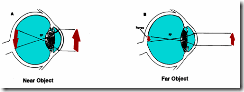
2.1 Accommodation allows the eye to focus on near objects
If the refractive index of the eye's lens system were fixed, then objects at only one given distance from the eye could produce sharply focused retinal images. It can be shown by simple geometrical optics that if, for instance, a distant object is brought closer to the eye, the image of this object would come into sharp focus in a plane behind that of the retina (resulting in a blurred image on the retina), unless the refractive power of the eye is increased. Although, most of the refractive power resides in the cornea, adjustments in the overall refractive power are done by the lens (i.e. the lens changes its shape, Fig. 6). In order to obtain sharp retinal images over a wide range of object distances, the eye has to be capable of modifying its refractive power according to object distance. This process, referred to as accommodation of the lens, consists of a reflex induced change in the thickness (curvature) of the eye's lens.
Normally (when looking at a distant environment) the elastic lens is held in a somewhat flattened shape by tension exerted on its periphery by radially oriented suspensory ligaments (zonal fibres) inserted in the sphincter of the radial ciliary smooth muscle fibres (Figs. 5 and 7). These ligaments pull the edges of the elastic lens capsule towards the surrounding ciliary body and by opposing the internal pressure within the elastic lens, keep it relatively flattened. When, in response to a conscious effort to fixate a near object, the ciliary sphincter muscle reflexly contracts, the diameter of its annulus around the lens decreases, the suspensory filaments slacken, and the radial tension around the circumference of the lens is released. The lens, due to its internal pressure, passively assumes a more spherical shape, resulting in an increased refractive power. Conversely, if an attempt is made to fixate a more distant object, the ciliary sphincter muscle relaxes, the diameter of its annulus around the lens increases, resulting in increased tension along the edges of the lens via the radial filaments. This in turn tends to flatten the lens and decrease its surface curvature, with consequent decrease in its refractive power. Thus, accommodation is a passive (elastic) response by the lens, as a consequence of a sphincter-like reflex action of the ciliary muscle. This reflex is under parasympathetic control.
In addition to lens accommodation, an attempt to fixate a near object is accompanied by (1) convergence of the two visual axis, so that both eyes are looking at the same object: and (2) constriction of the pupils, primarily due to an increased parasympathetic drive to the circular muscles of the iris diaphragm. As in the case of a camera, a smaller aperture gives a greater depth of focus, and results in reduced image distortion.
Figure 7. The Mechanism Mediating Changes Lens Shape
In summary then, the Accommodation Reflex consists of:
(1) change in the refractive power of the lens.
(2) vergence movements of the eyes (convergence).
(3) change in the size of the pupil.
2. 2 Errors in Refraction
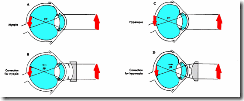
The normal (emmetropic) eye can focus parallel light rays reflected from a distant object sharply on the retina (Fig. 6) while the ciliary sphincter muscle is relaxed (i.e. no conscious effort is made to fixate a particular object).
In the myopic eye (Fig 8: left), with the ciliary sphincter relaxed, parallel light rays from a distant object come into focus in a plane in front of the retina, resulting in a blurred retinal image. No mechanism exists to decrease the refractive index of the eye when the ciliary muscle is completely relaxed. Therefore distant objects can not be brought into focus on the retina, but as an object comes closer it can eventually be brought into focus. This condition, commonly referred to as "near-sightedness", may be due to either the lens having excessive refractive power, or an excessively elongated eye ball. In either case, the myopic condition can be remedied by placing a concave lens of appropriate refractive power in front to the eye (a concave lens causes the light rays entering the eye to diverge, thereby effectively shifting the focal plane further back).
In a hypermetropic eye (Fig 8: right), with the ciliary sphincter relaxed, parallel light rays from a distant object come into focus in a plane in behind of the retina. This condition, commonly referred to as "far-sightedness", may be due to either having a lens of insufficient refractive power or an eye ball that is too short. A hypermetropic eye can bring distant objects into focus by a conscious effort of accommodation, i.e. constriction of the ciliary sphincter with a resulting increase of lens refractive power. However, close objects will remain blurred because in the hypermetropic eye near accommodation is severely limited. This condition can be remedied by placing a convex (i.e. converging) lens in front of the eye.
Note that in both the myopic and hypermetropic eye, the lens is capable of accommodation, except that the required range is limited in the far or near direction, respectively. With advancing age, the elasticity of the lens decreases, with the result that it progressively loses its accommodative properties, and hardens into a flattened shape. This results in presbyopia, a condition where only distant objects can be resolved. Again, this condition can be readily corrected with appropriate extraocular lenses.
Figure 8. The Myopic (near-sighted) and Hypermetropic (far-sighted) Eye
2.3 Visual Acuity
The optical apparatus of the eye is designed to produce a good sharp unblurred image. The question arises: "How good an image can the visual system actually produce?" This question raises the issue of visual acuity. Visual acuity is the ability to discriminate fine detail. it is defined as the reciprocal of the minimum resolvable visual angle (in minutes of arc) subtended by the eye.
Minimum Angle Acuity
2 min. 0.5
1 min. 1.0
30 sec. 2.0
Visual acuity is defined in terms of the visual angle subtended at the eye, rather than in terms of absolute object size. This is because object size and its projected distance are reciprocally related: an object moved to twice a given distance from the eye will project a retinal image half the size. Thus, in Fig. 9, an object of height h subtends a visual angle a at a distance d; to appear the same height at distance 2d, it would have to increase to twice the height (2h), thereby retaining the same angular intercept a.
Figure 9. The Definition of "Visual Angle"
A convenient illustration of this principle is to be found in observing a picture in the news paper. Looked at closely, it may appear to be composed of a series of small dots; observed from a distance, these dots will merge into an un-resolvable grayish surface: By increasing the distance from the eye, while keeping the linear distance between the dots fixed, the minimal visual angle between the dots has fallen below the threshold of resolution (0.5 - 1.0 minutes of arc for normals). The precise value in a given instance depends on light intensity, degree of pupillary constriction, pattern type, colour, and several other factors.
Clinically, visual acuity is tested and defined in terms of the minimum distance that two lines must be separated in order to appear distinct. This minimum distance is measured by using a Snellen letter chart (Fig. 10A, 1/4 actual size). The sizes of the letters in this chart are designed so that a normal person should be able to read the vertical letter size in the smallest row of letters at a distance of 6 meters (20 ft). If a patient can correctly read down all the letters to the smallest row, then he /she is said to have normal 6/6 (or 20/20) vision. If, however, a patient can read at 6 m only a letter size that a normal person can read a 9 m, then he/she is rated at the lower score of 6/9 (or 20/30).
Figure 10. The Snellen Chart
After passing through the optical filters of the cornea and the lens, light rays impinge upon the retina where they from a two dimensional image of the outside world.
3.1 How can the retina operate over a very wide range of light intensities
The question arises: How much light energy is needed to convert this optical image into neural information? The normal human eye is capable of operating over a wide range of light intensities, from dim moonlight to bright sunshine (a range of about 1:1010). Under appropriate conditions, receptor cells in the retina can detect a single quantum of light. How does the retina combine this exquisite (the maximum sensitivity that is theoretically possible) sensitivity to a single photon, with its enormous intensity range of operation? or, in other words, how does the eye maintain an adequate sensitivity to a given small change in illumination despite exposure to widely differing levels of mean light intensities? The visual system employs two distinct mechanisms 1) The light reflex and 2) dark adaptation to deal with this problem.
3.1 A.) The Light Reflex
Increased intensity of light on the retina activates a neural reflex mechanism that causes the pupil to constrict (miosis) via contraction of the circular (sphincter-like) muscles of the iris diaphragm. This reflex is mediated via parasympathetic pathways of the autonomic nervous system. Conversely, light reduction leads to pupillary dilation (mydriasis; contraction of the radial muscles of the iris), through the action of sympathetic autonomic pathways. The light reflex is consensual, i.e. light shone into one eye causes pupillary adjustments simultaneously in both eyes. The total range of pupillary diameter adjustment via the light reflex ( 2 - 8 mm) results in a total change of retinal illumination by a factor of only about 16. This is far less than the potential 1010 range which the visual can handle.
Due to its characteristic innervation, the light reflex may serve as a useful clinical diagnosis tool. Intoxication with alcohol or morphine is characterized by marked pupillary constriction (miosis); while the effects of cocaine are the reverse (mydriasis). Because the light reflex and accommodation reflex (discussed above) are mediated by different neural pathways, these two pupillary reflexes are affected differently by disease processes. For example, tertiary syphilis (tabes) is frequently characterized by the absence of a light reflex and a highly constricted pupil (pin-point or Argyll-Robinson pupil), but near normal adjustments of the pupil during the accommodation reflex.
Conversely, certain forms of neuropathy are characterized by the absence of pupillary accommodation, but a near normal light reflex.
Figure 11. The Dark Adaptation Curve
3.1 B.) Dark Adaptation
This mechanism operates over a much wider range of light intensities. It is a common experience that, upon entering a dark room from the sun-lit exterior, one’s initial visual perception is severely impaired. It is only after a few minutes, that one begins to discern visual contours. This phenomenon can be described more precisely by taking a human subject from day light into a darkened room, and testing his/her threshold of vision (every minute) with a small light source whose intensity is adjustable. Every minute (for a total time period of 40 minutes, in this example) the experimenter would briefly expose the subject to the light and adjust its brightness down to the level that the subject can just barely detect. Typical results from such a hypothetical experiment are plotted in Fig. 11.
A number of features of this graph deserve attention:
(1) The initial retinal sensitivity (at time = 0) is arbitrarily designated as 8. After approximately 1/2 hour in the dark, the subject’s sensitivity to light has increased by a factor of 106 (represented by a decrease in log10 threshold from 8 to 2). (Note, in Figure 11 threshold is plotted using a relative intensity scale. The actual value of the threshold intensity will depend on the specific test conditions and is typically measured in units (such as trolands) which take into account the luminance of the light source (candles/m2) and the area of the pupil (mm2).
(2) There is an initial rapid decrease in the threshold intensity which asymptotes towards a plateau about 100 times (102) lower than starting value. After this initial 7-8 minute period, there is a sudden abrupt break in the flattened curve, starting a second episode of more gradual decrease in threshold intensity, which eventually (after 30-40 minutes) approaches its final maximum value.
The functional significance of this dark adaptation is quite clear: it allows the eyes to operate adequately over a range of light intensities comprising many orders of magnitude (over a billion-fold). By comparison, the mechanical action of the iris diaphragm is capable of modifying the amount of light entering the eye by a factor of about 16. In order to understand the mechanisms which underly this remarkable adaptive response, it is necessary to consider the transduction process (light energy- neural activity) of the eye.
Figure 12. The Visible Spectrum
3.2 Rods and Cones: The receptor cells
The human eye is a receptor organ for electromagnetic radiation. The eye is sensitive to only a very small fraction of (.0001 %) of the overall electromagnetic frequency spectrum. This is illustrated by the blackened region of the spectrum in Fig. 12. The visible spectrum is sandwiched between the very short wave-length (high frequency) of X-radiation and the longer wavelengths (lower frequencies) of the radio broadcast bands.
The human retina contains two types of light sensitive receptor cells: cones (concentrated primarily in the central region of the fovea), and rods (largely in the periphery). Both types of cells contain light sensitive molecules referred to as photopigments in their outer segments (Fig. 13). The type of pigment differs for different cells: all rods contain rhodopsin, whereas cones can be divided into three groups (1) those containing erythrolabe, (2) those containing chlorolabe, and (3) those containing cyanolabe. Rhodopsin is sensitive to a broad range of spectral frequencies, while the three cone pigments are optimally sensitive to light frequencies associated with the three primary colours red, green and blue, respectively. The cones form the basis for colour vision.
All four photopigments have a similar chemical composition. They consist of a protein (opsin) and a chromophore (retinene) which is structurally related to vitamin A. A schematic of the chain of events which occur in a rod photoreceptor is illustrated in Fig. 13. Light energy (photons) act upon the chromophore which is then spilt away from the opsin moiety. This latter reaction activates a phosphodiesterase (an enzyme which hydrolizes cGMP); which in turn transiently blocks Na+ channels in the cell membrane, reducing the inward Na+ current which normally occurs in the dark (the dark current). This results in a change in membrane potential (a receptor potential). The receptor potential induces neural impulses in the optic nerve which projects to the brain via a class of interneurons called bipolar cells. Two other retinal cell types (horizontal and amacrine cells) also contribute to the processing of the visual signal before it leaves the retina via the axons of the retinal ganglion cells which constitute the optic nerve.
Figure 13. The Electromagnetic-Neural Transduction Process
The light sensitivity of individual retinal receptor cells is roughly proportional to the antilogarithm of the concentration of intact photopigment molecules. As a consequence of pigment loss via the biochemical molecule splitting reactions, exposure of the retina to light results not only in the energy transducing process, but simultaneously in an overall rapid reduction in the sensitivity to light (light adaptation).
This functionally important process of light adaptation can be considered, from the chemical point of view, as merely a loss of dark adaptation (described above). In fact, in bright light, the rate of rhodopsin break down is so rapid that rods become visually ineffective. This is not the case for cones which operate optimally in bright light. Thus, exposure to a dimly lit, or dark environment will result in pigment reconstitution (a recombination of the retinal and opsin fragments).
These properties of rods and cones can be used to better understand the results which were described above in Fig. 11. The overall decrease in threshold is clearly related to the process of overall pigment reconstitution. The initial rapid decrease towards the 100 X plateau results from the regeneration of cone pigments , since cones rapidly regain their optimal sensitivity. The optimal threshold of cones, however is still several orders of magnitude above the optimal (single photon ) threshold of rods, which is attained only after a prolonged (30-40 minute) sojourn in the dark. Consequently, the retina contains two separate visual systems:(1) a peripheral system consisting of high sensitivity (low threhold), slowly adapting rods - subserving exclusively (colourless) night vision and (2) a foveal system consisting of low sensitivity (high threshild) fast adapting cones- subserving day time and colour vision.
3.3 Spectral sensitivities of rods and cones
In addition to differences in sensitivities and rates of dark adaptation, the two systems also differ in their spectral responses. This is shown by the results of a hypothetical experiment illustrated in Fig. 14. In this experiment, as in that illustrated in Fig. 11, a dark adapted subject is tested. However in this experiment, the researcher exchanges the white light source used in the previous experiment for one which produces variable intensity radiation at different wavelengths (i.e. colours in the visible spectrum 400 - 700 mm ). For each wavelength, the experimenter again determines the subject’s threshold of vision. The results plotted as relative retinal sensitivity(1/threshold) against wavelength, would look somewhat like curve B (scotopic curve) in Fig. 14. The peak sensitivity is at 500 mm (blue green), with a symmetrical fall off towards both the blue and red portions of the spectrum. In addition, the subject would report that the test light regardless of its apparent brightness, always appeared subjectively as a shade of gray. Thus, although the dark-adapted (peripheral) retina is differentially sensitive to different wavelengths of light, it does not mediate colour perception. The rod system is colour blind (i.e. a red rose on a green lawn, seen in dim moonlight from the corner of ones eye, would appear as a black blob on a dark gray background).
If the experimenter then performs a similar experiment of the subject who is in the light adapted condition(i.e. normal day light). The test would yield a similar bell shaped sensitivity curve (Fig. 14, curve C, the photopic curve), but its peak would now be at 550 mm (the green-yellow range of the spectrum). Please note, retinal sensitivity along the ordinate in Fig. 14 is a relative (percentage) scale, such that in absolute terms, the maximum photopic sensitivity would only be a fraction of scotopic sensitivity. Furthermore, the subject would report that he/she could distinguish the colours associated with the test lights of different wavelengths, and that the threshold intensities could be distinguished primarily through foveal vision. This change in the spectral sensitivity of the eye which occurs as a function of lighting condition is referred to as the Purkinje Shift.
Figure 14. The Spectral Sensitivities of the Eye Depends on Lighting Conditions
4.1 The Rationale for colour vision
The dim image of a moonlit world having been brought to focus at the plane of the retina by the optical apparatus of the dark adapted eye, will usually allow for satisfactory discrimination of large patterns and contours. However, visual perception under these scotopic conditions will usually be limited by two factors: (1) poor visual acuity, due to the absence of cone-mediated processes (i.e. poor resolution) and (2) the lack of colour perception: Objects are discriminated from each other and the background based on differences in shades of gray. This latter condition is satisfactory for animals that operate largely at night time, where the high sensitivity to modest light inputs is more important than either high visual acuity or colour perception.
The question, then arises: Why has evolutionary pressure given way to colour vision? The cause is clear: Colour vision provides an enhanced ability to distinguish the contours and shapes of objects. The subjective experience of colour can be broken down into three semi-independent sensibilities: (1) Hue (ordinarily referred to as colour) is the sensation by which different parts of the electromagnetic spectrum are distinguished (2) Saturation (or purity) reflects the degree to which a given hue has been diluted by a neutral grey, and (3) Brightness (intensity) is a sensation that is shared with achromatic visual mechanisms and is related to the total amount of light that is intercepted by the eye.
Photopic vision allows for 500 distinguishable steps of brightness discrimination for every combination of hue and saturation. In addition to that, the normal eye can distinguish up to some 200 different hues, and their are some 20 distinguishable steps of saturation for each hue. Consequently, by utilizing differences in wavelength, in addition to differences (gradients) of light energy, the available number of criteria for object discrimination has been substantially increased from about 500 to a value of about 500 X 200 X 20 or 2 X 106 .
4.2 Coding for Colour in the Nervous System
Although the physical stimulus for colour (hue) perception depends on the physical parameters (wavelength) of light, the subjective experience is rooted in the physiological properties of the eye and higher level visual pathways. The first question which arises is, How is the message that allows a normal human to differentiate 200 odd Hues conveyed by the peripheral receptors? Are there 200 different types of cones?
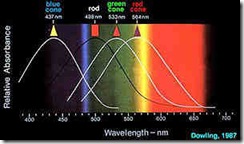
Evolution solved this problem in most vertebrate eyes with three sets of cones. Each cone contains a distinct light sensitive chemical (pigment), with one type of pigment sensing predominately blue (260 mm ), another predominately green (530 mm ) and the third predominately a red ( actually reddish-yellow, 580 mm ). The relative sensitivities for these light absorbtion spectra for these primary colour receptors are shown in Fig. 15. The summed envelope of theses curves is equivalent to the eye’s photopic response curve (see Fig. 14). The subjective perception of colour then involves not simply the action of a single class of cone receptors, but the interaction between their responses.
For example, a stimulus which consists of a pure 600 mm wavelength light will normally be perceived as "orange", and it can be seen from Fig. 15 that it will excite both the red and green cone populations. It is this ration of red-green cone excitation (with, in this case, no contribution from the blue cone mechanism) which the central nervous system interprets as the hue "orange". All other perceived hues are similarly the result of the proportional activation of all three cone mechanisms by selective wavelengths of light.
Figure 15. The Relative Sensitivity of the Rod and the Three Human Cone Types
4.3 Colour Blindness
Colour blindness is a genetic disease affecting predominately males (8% males, 0.4 females in the Caucasian population). It is inherited as a recessive X-linked trait. Most forms of colour blindness are due to the absence or impairment, of one or two cone systems. It is customary to divide colour vision disorders into those in which three, two or one cone system is used to generate all subjective impressions of colour hue.
Subjects who have one or several of the cone systems impaired (but not missing) are trichromats since they are still able to use three cone systems (albeit anomalous ones) to generate aspects of colour vision. Dichromats are subjects with one cone system completely missing. They rely on the two remaining systems for rudimentary colour vision. Monocrats are subjects who lack two of the three normal cone systems.
The terminology used to describe these deficits uses "protos" (meaning "first" in Greek) for the red cones, "deuteros" for the green cones (meaning "second" in Greek) , and "tritos" for the blue cons (meaning "third" in Greek). Accordingly, Trichromats may suffer from Protanomaly, Deuteranomaly, or Tritanomaly (i.e. the impairment of the red, green or blue cone systems); while Dichromats will be affected by Protanopia, Deuteranopia, or Tritanopia (i.e. the absence or non-function of the red, green, or blue cone systems).
Anomalies of Colour Vision
Cone System Trichromats Dichromats Monochromats
RED
(protos) Protanomaly Protanopia
GREEN
(deuteros) Deuteranomaly Deuteranopia
BLUE
(tritos) Tritanomaly Tritanopia






























+ comments + 2 comments
I have been wondering for some time the layout of the rod/and cones, I hypothesize they are laid out in natures typical pattern; spirals in a similar way to pine cones or sunflowers. Google+ +Trevor Seaman
I am an Optometry student writing an essay on light/dark adaptation and have used some ideas from this article. I would be grateful if you could tell me the name of the author, date of publication and Producer/publisher. Thank you in advance.
Post a Comment